Shiseido to Begin Cell Hair Regeneration Trial
by Kevin Rands | August 11, 2016 8:17 pm
Shiseido recently announced that they are moving forward with a study that will involve 60 men and women with thinning hair. This trial will take place at the Tokyo Medical University Hospital and Toho University, Ohasi Medical Center.
The method they plan to study is based on a new hypothesis in hair regeneration. For many years scientists have evaluated ways to multiply the number of hair follicles, or stimulate native hair follicles using cellular methods. Terms like Hair Multiplication, Follicle Cloning, and similar may ring a bell. But no matter the method, the goal is the same: The ability to create new hairs where there were only few. If successful, having access to an unlimited amount of your own hair (whether it needs to be implanted surgically, or injected on a cellular level) could mean a practical cure for hair loss. They will be evaluating both safety, and effectiveness based on hair fiber thickness and hair counts over a period of 3 years.
RCH-01 Hair Regeneration Technology
Shiseido acquired the exclusive license of Canadian bio-venture company Replicel back in July 2013 to use their hair regeneration technology “RCH-01” in the entire Asian region. Due to Japan’s unique regenerative medicine regulatory environment we might see this technology launched before 2020 on the Japanese market, sooner than anywhere else in the world.
RCH-01 technology is based on the research of Kevin J. McElwee et al1[1]. It is a form of autologous cell therapy[2] based on dermal sheath cup cells. These specific kind of cells, which are located in the hair follicle, will be extracted from the back of the head, just like during a first stages of a typical hair transplant procedure. They will extract a 4-8 millimeter punch biopsy which will then be “multiplied” in the lab for a period of 3 months. After the successful replication of these cells, they will be injected back into the scalp.
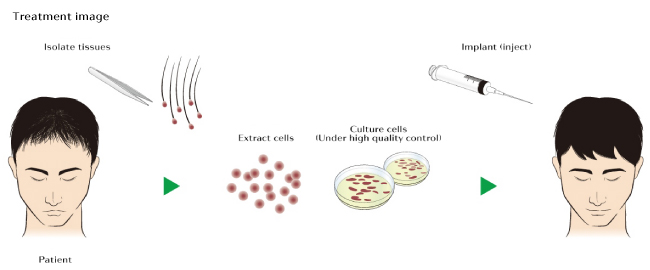
Replicel itself has already conducted a phase 1 clinical trial.[3] While they only managed to increase hair density by about 10% in 70% of the participants, this was still a first step in the right direction. It was also primarily a safety test so they bypassed some important steps including repeat injections, dose analysis, and using a sufficiently large treatment area. These are all very influential factors that Shiseido can play around with during the upcoming 3 year trial in Japan.
Replicating cells is only part of the process. They will then be injected back into the scalp, where they hope to literally grow a new crop of hair. So the success of this upcoming trial will rely heavily on how these cells behave when injected. Will they secrete stimulative factors and cause temporary thickening of existing hair follicles? Or will they incorporate into the hair follicle structure itself and repopulate the dermal papilla? If it is the latter, the treatment may be a huge breakthrough with very long lasting or permanent effects.
Dermal Papilla’s Role in Hair Loss
To understand this a bit more we need to take look at the hair follicle.
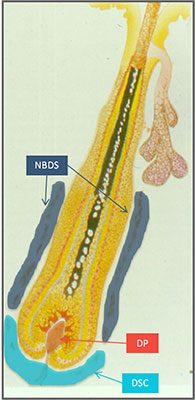
The base of the hair follicle is where the “dermal papilla” is located. This compartment of cells are crucial for the hair follicle, and this has been shown in many studies. It plays a significant role in all phases of the follicle life cycle from before its beginning to the cycling out of growth.
Back in 1958 a study showed that the thickness of a hair is controlled by the amount of dermal papilla cells in that hair follicle2[4]. In androgenetic alopecia[5] (typical male and female pattern hair loss) we see that the dermal papilla is diminished heavily too.
A recent study in 2013 showed that the removal of dermal papilla cells causes a type of follicular decline3[6] that matches the thinning and miniaturization process of androgenetic alopecia. The dermal papilla is also the main site of androgen action4[7]. The place where DHT does its dirty work, making your life miserable by giving you bad hair days for the rest of your life. The androgens Testosterone and Dihydrotestosterone bind to the androgen receptor located at the surface of these very cells.
Another study showed that bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks progenitor cells5[8]. Progenitor cells are controlled by, and dependent on dermal papilla cells6[9],7[10].
This means that the Dermal Papilla is of huge importance in hair growth. And it is no surprise that repopulating the dermal papilla in Androgenetic Alopecia may reverse miniaturization. Likewise, replacing dermal papilla cells may pretty much make someone immune to the effects of hair loss!
Sheath Cup Cells and Dermal Papilla
So is this RCH-01 technology based on dermal papilla cells? Nope. Its based on sheath cup cells instead. And the reason for this is simple: it has been hypothesized that dermal papilla cells themselves come from somewhere else in the hair follicle. And Replicel believes that mystery source may be dermal sheath cup cells.
However if the injected dermal sheath cup cells will just release stimulative factors and temporary stimulate native hair follicles then it remains to be seen if the treatment will be good enough for Shisheido. Who knows though, perhaps repeated dosages that simply stimulate the hair follicles will show very good results too. I guess we will have to wait for the data which will give the final answer.
References:
- McElwee KJ1, Kissling S, Wenzel E, Huth A, Hoffmann R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003 Dec;121(6):1267-75.
- VAN SCOTT EJ, EKEL TM. Geometric relationships between the matrix of the hair bulb and its dermal papilla in normal and alopecic scalp. J Invest Dermatol. 1958 Nov;31(5):281-7.
- Chi W1, Wu E, Morgan BA. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development. 2013 Apr;140(8):1676-83.
- Randall VA1, Hibberts NA, Thornton MJ et al. The hair follicle: a paradoxical androgen target organ. Horm Res. 2000;54(5-6):243-50.
- Garza LA1, Yang CC, Zhao T et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest. 2011 Feb;121(2):613-22.
- Morgan BA. The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb Perspect Med. 2014 Jul
- Clavel C, Grisanti L, Zemla R et al. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev Cell. 2012 Nov 13;23(5):981-94.
- 1: #1
- autologous cell therapy: https://en.wikipedia.org/wiki/Autologous_stem-cell_transplantation
- phase 1 clinical trial.: http://replicel.com/wp-content/uploads/2015/02/Poster-WCHR-2013-AGA-treatment.pdf
- 2: #1
- androgenetic alopecia: https://www.hairlosstalk.com/hair-loss-treatments/
- 3: #1
- 4: #1
- 5: #1
- 6: #1
- 7: #1
Source URL: https://www.hairlosstalk.com/news/new-research/shiseido-stem-cell-regeneration/