DHT trigger an inflammation cascade which turn chronic.
DHT promotes VCAM-1 expression & activation of NF-KappaB. NF-Kappab is basically the on/off switch for chronic inflammation problems and diseases.
http://en.wikipedia.org/wiki/VCAM-1
The VCAM-1 protein mediates the adhesion of
lymphocytes,
monocytes,
eosinophils, and
basophils to vascular
endothelium. It also functions in leukocyte-endothelial cell
signal transduction, and it may play a role in the development of
atherosclerosis and
rheumatoid arthritis.
Upregulation of VCAM-1 in endothelial cells by cytokines occurs as a result of increased
gene transcription (e.g., in response to
Tumor necrosis factor-alpha (TNF-α) and
Interleukin-1 (IL-1)) and through stabilization of
Messenger RNA (mRNA) (e.g.,
Interleukin-4 (IL-4)). The promoter region of the VCAM-1 gene contains functional tandem
NF-κB (nuclear factor-kappa B) sites. The sustained expression of VCAM-1 lasts over 24 hours.
http://en.wikipedia.org/wiki/NF-κB
Because NF-κB controls many genes involved in inflammation, it is not surprising that NF-κB is found to be chronically active in many inflammatory diseases, such as inflammatory bowel disease, arthritis, sepsis, gastritis, asthma, atherosclerosis[SUP]
[56][/SUP] and others. It is important to note though, that elevation of some NF-κB inhibitors, such as
osteoprotegerin (OPG), are associated with elevated mortality, especially from
cardiovascular diseases
Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-kappaB-dependent pathway.
Death AK,
McGrath KC,
Sader MA,
Nakhla S,
Jessup W,
Handelsman DJ,
Celermajer DS.
Author information
Abstract
There exists a striking gender difference in atherosclerotic vascular disease. For decades, estrogen was considered atheroprotective; however, an alternative is that androgen exposure in early life may predispose men to earlier atherosclerosis. We recently demonstrated that the potent androgen, dihydrotestosterone (DHT), enhanced the binding of monocytes to the endothelium, a key early event in atherosclerosis, via increased expression of vascular cell adhesion molecule-1 (VCAM-1). We now show that DHT mediates its effects on VCAM-1 expression at the promoter level through a novel androgen receptor (AR)/nuclear factor-kappaB (NF-kappaB) mechanism. Human umbilical vein endothelial cells were exposed to 4-400 nm DHT. DHT increased VCAM-1 mRNA in a dose- and time-dependent manner. The DHT effect could be blocked by the AR antagonist, hydroxyflutamide. DHT increased VCAM-1 promoter activity via NF-kappaB activation without affecting VCAM-1 mRNA stability. Using 5' deletion analysis, it was determined that the NF-kappaB sites within the VCAM-1 promoter region were responsible for the DHT-mediated increase in VCAM-1 expression; however, coimmunoprecipitation studies suggested there is no direct interaction between AR and NF-kappaB. Instead, DHT treatment decreased the level of the NF-kappaB inhibitory protein. DHT did not affect VCAM-1 protein expression and monocyte adhesion when female endothelial cells were tested. AR expression was higher in male, relative to female, endothelial cells, associated with increased VCAM-1 levels.
These findings highlight a novel AR/NF-kappaB mediated mechanism for VCAM-1 expression and monocyte adhesion operating in male endothelial cells that may represent an important unrecognized mechanism for the male predisposition to atherosclerosis.
Dihydrotestosterone stimulates cerebrovascular inflammation through NFkappaB, modulating contractile function.
Author information
Abstract
Our previous studies show that long-term testosterone treatment augments vascular tone under physiological conditions and exacerbates endotoxin-induced inflammation in the cerebral circulation. However, testosterone can be metabolized by aromatase to estrogen, evoking a balance between androgenic and estrogenic effects. Therefore, we investigated the effect of the nonaromatizable androgen receptor agonist, dihydrotestosterone (DHT), on the inflammatory nuclear factor-kappaB (NFkappaB) pathway in cerebral blood vessels. Cerebral arteries were isolated from orchiectomized male rats treated chronically with DHT in vivo. Alternatively, pial arteries were isolated from orchiectomized males and were exposed ex vivo to DHT or vehicle in culture medium. DHT treatment, in vivo or ex vivo, increased nuclear NFkappaB activation in cerebral arteries and increased levels of the proinflammatory products of NFkappaB activation, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS).
Effects of DHT on COX-2 and iNOS were attenuated by flutamide. In isolated pressurized middle cerebral arteries from DHT-treated rats, constrictions to the selective COX-2 inhibitor NS398 or the selective iNOS inhibitor L-nil, [L-N6-(Iminoethyl)lysine], were increased, confirming a functional consequence of DHT exposure
. In conclusion, activation of the NFkappaB-mediated COX-2/iNOS pathway by the selective androgen receptor agonist, DHT, results in a state of vascular inflammation. This effect may contribute to sex-related differences in cerebrovascular pathophysiology.
Fibrosis is the formation of excess fibrous
connective tissue in an organ or tissue in a reparative or reactive process. This can be a reactive, benign, or pathological state. In response to injury this is called
scarring and if fibrosis arises from a single cell line this is called a
fibroma. Physiologically this acts to deposit connective tissue, which can obliterate the architecture and function of the underlying organ or tissue. Fibrosis can be used to describe the pathological state of excess deposition of fibrous tissue, as well as the process of connective tissue deposition in healing.[SUP]
[1][/SUP]
Fibrosis is similar to the process of scarring, in that both involve stimulated cells laying down
connective tissue, including
collagen and
glycosaminoglycans. Immune cells called
Macrophages, and damaged tissue between surfaces called
interstitium release TGFbeta. This can be because of numerous reasons, including inflammation of the nearby tissue, or a generalised inflammatory state, with increased circulating mediators. TGFbeta stimulates the proliferation and activation of
fibroblasts, which deposit connective tissue.[SUP]
[2]
[/SUP]
http://en.wikipedia.org/wiki/Fibrosis
Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis
Satoshi Ueha[SUP]1,2[/SUP],
Francis H. W. Shand[SUP]1,3[/SUP] and
Kouji Matsushima[SUP]1,2[/SUP]*
- [SUP]1[/SUP] Department of Molecular Preventive Medicine, Graduate School of Medicine, The University of Tokyo, Bunkyo-ku, Tokyo, Japan
- [SUP]2[/SUP] Japan Science and Technology Agency, Core Research for Evolutional Science and Technology, Tokyo, Japan
- [SUP]3[/SUP] Departmhttp://www.hairlosstalk.com/interact/showthread.php/69374-New-Dermaroller-Study-Thoughts-comments/page386ent of Pharmacology, University of Melbourne, Melbourne, VIC, Australia
Organ fibrosis is a pathological condition associated with chronic inflammatory diseases.
In fibrosis, excessive deposition of extracellular matrix (ECM) severely impairs tissue architecture and function, eventually resulting in organ failure. This process is mediated primarily by the induction of myofibroblasts, which produce large amounts of collagen I, the main component of the ECM. Accordingly, the origin, developmental pathways, and mechanisms of myofibroblast regulation are attracting increasing attention as potential therapeutic targets. The fibrotic cascade, from initial epithelial damage to eventual myofibroblast induction, is mediated by complex biological processes such as macrophage infiltration, a shift from Th1 to Th2 phenotype, and by inflammatory mediators such as transforming growth factor-β. Here, we review the current understanding of the cellular and molecular mechanisms underlying organ fibrosis.
http://www.frontiersin.org/Journal/1...00071/abstract
Folliculitis is defined histologically as the presence of inflammatory cells within the wall and ostia of the hair follicle, creating a follicular-based pustule. The actual type of inflammatory cells can vary and may be dependent on the etiology of the folliculitis, the stage at which the biopsy specimen was obtained, or both. The inflammation can be either limited to the superficial aspect of the follicle with primary involvement of the infundibulum or the inflammation can affect both the superficial and deep aspect of the follicle. Deep folliculitis can eventuate from chronic lesions of superficial folliculitis or from lesions that are manipulated, and this may ultimately result in scarring.
Perifolliculitis, on the other hand, is defined as the presence of inflammatory cells in the perifollicular tissues and can involve the adjacent reticular dermis. Folliculitis and perifolliculitis can manifest independently or together as a result of follicular disruption and irritation.
This is a good evidence of chronic inflammation and shows how deep the problem is.
Two 4 mm punch biopsies were performed on the occiput of the 7 controls and the wound was closed with 3-0 polyglactin 910.
Hair transplant strip showing marked perifollicular fibrosis

Evaluation of perifollicular inflammation of donor area during hair transplantation in androgenetic alopecia and its comparison with controls
Background: Mild perifollicular inflammation is seen in both androgenetic alopecia (Androgenetic Alopecia) cases and normal controls, whereas moderate or dense inflammation with concentric layers of collagen, is seen in Androgenetic Alopecia cases but only in very few normal controls, and may lessen the response to topical minoxidil. Moderate or dense lymphocytic inflammation and perifollicular fibrosis have poor hair growth following transplantation.
Aim: The purpose of the study is to evaluate the perifollicular lymphocytic inflammation and fibrosis in Androgenetic Alopecia patients during follicular unit hair transplantation (FUT) and its comparison in normal controls.
Materials and Methods: A total of 21 male patients with Androgenetic Alopecia and 7 matched controls participated in the study. Histopathological analysis of biopsy specimens from donor strip of patients during the hair transplantation and two 4 mm punch biopsies on controls were performed. Morphometric analysis was performed and perifollicular fibrosis was scored based on the width of the condensed collagen at the lower infundibulum and isthmus from 0 to 3. Perifollicular infiltrate was also scored 0-3 and a total score of 3 or more out of 6 was considered significant.
Results: Nearly 76% of Androgenetic Alopecia patients had perifollicular fibrosis more than 50 μm at ×200 magnification. Almost 33.33% patients had moderate/dense perifollicular lymphocytic infiltrate whereas none of the controls had it. Total score in Androgenetic Alopecia cases was significantly higher than controls (
P = 0.012) using Chi-square test.
Out of 21 patients, 13 had a score of 3 or more and were followed-up with monthly treatment with intralesional steroids using a dermaroller. Conclusion: Histopathological evaluation of the donor area is a must during hair transplantation to evaluate the extent of perifollicular inflammation and achieve better results by following it up with treatment directed to decrease the inflammation.
They are achieving better regrowth with topical anti-inflammatory during hair transplant..This is indeed, really interesting.
http://www.ijtrichology.com/article....st=Nirmal#ref6
Quantitative and ultrastructural analysis of inflammatory infiltrates in male pattern alopecia.
Sueki H,
Stoudemayer T,
Kligman AM,
Murphy GF.
Author information
Abstract
In order to determine whether lymphocytic inflammation around the lower infundibula in male pattern alopecia is incidental or a general phenomenon, we performed morphometric and ultrastructural analysis of inflammatory infiltrates in the transitional zones of the vertex and occipital hairy scalps of 19 patients with male pattern alopecia. Six normal subjects served as controls. The number of inflammatory infiltrates around the follicular infundibula of the alopecic vertices and non-alopecic occiputs of male pattern alopecia patients was significantly greater than the corresponding control value. The number of mast cells in the widened fibrous tracts in the vertices of male pattern alopecia patients was significantly greater than those in the adventitial fibrotic sheaths of control subjects and the non-alopecic occiputs of male pattern alopecia patients.
These data support the idea that the inflammatory process may be, at least in part, responsible for the development of male pattern alopecia.
Article: Perifollicular fibrosis: pathogenetic role in androgenetic alopecia.
Source: Biol Pharm Bull. 2006 Jun;29(6):1246-50.
Author(s): Yoo HG, Kim JS, Lee SR, Pyo HK, Moon HI, Lee JH, Kwon OS, Chung JH, Kim KH, Eun HC, Cho KH
Department of Dermatology, Seoul National University College of Medicine, Laboratory of Cutaneous Aging and Hair Research, Clinical Research Institute, Seoul National University Hospital, and Institute of Dermatological Science, Seoul National University. |
Summary:
Fibrosis is a scarring process in the skin that can damage the hair follicle (hair loss). This study shows that increased Testosterone speeds up fibrosis while treatment with Finasteride helps slow fibrosis. Stopping or slowing fibrosis may be another method by which Finasteride may help prevent hair loss.
Androgenetic alopecia (Androgenetic Alopecia) is a dihydrotestosterone (DHT)-mediated process, characterized by continuous miniaturization of androgen reactive hair follicles and accompanied by perifollicular fibrosis of follicular units in histological examination. Testosterone (T: 10(-9)-10(-7) M) treatment increased the expression of type I procollagen at mRNA and protein level. Pretreatment of finasteride (10(-8) M) inhibited the T-induced type I procollagen expression at mRNA (40.2%) and protein levels (24.9%). T treatment increased the expression of transforming growth factor-beta 1 (TGF-beta1) at protein levels by 81.9% in the human scalp dermal fibroblasts (DFs). Pretreatment of finasteride decreased the expression of TGF-beta1 protein induced by an average of T (30.4%). The type I procollagen expression after pretreatment of neutralizing TGF-beta1 antibody (10 mug/ml) was inhibited by an average of 54.3%. Our findings suggest that T-induced TGF-beta1 and type I procollagen expression may contribute to the development of perifollicular fibrosis in the Androgenetic Alopecia, and the inhibitory effects on T-induced procollagen and TGF-beta1 expression may explain another possible mechanism how finasteride works in Androgenetic Alopecia.
http://www.derma-haarcenter.ch/files...+Barcelona.pdf
|
Cosmet Dermatol. 2009 Jun;8(2):83-91
Androgenetic alopecia in males: a histopathological and ultrastructural study.
El-Domyati M, Attia S, Saleh F, Abdel-Wahab H.
Department of Dermatology, Faculty of Medicine, Al-Minya University, Al-Minya, Egypt.
Background Androgenetic alopecia is a common cosmetic hair disorder, resulting from interplay of genetic, endocrine, and aging factors leading to a patterned follicular miniaturization.
Microinflammation seems to be a potential active player in this process. Aims To study the histopathological and ultrastructural changes occurring in male androgenetic alopecia (Androgenetic Alopecia). Patients/methods Fifty-five subjects were included in this study (40 with Androgenetic Alopecia and 15 as normal age-matched controls). Skin biopsies from frontal bald area and occipital hairy area were subjected to histopathological examination, immunohistochemical staining for collagen I and ultrastructural study. Results The frontal bald area of patients showed highly significant increase in telogen hairs and decrease in anagen/telogen ratio and terminal/vellus hair ratio (P < 0.001).
Perifollicular inflammation was almost a constant feature in early cases and showed a significant correlation with perifollicular fibrosis (P = 0.048), which was more marked with thickening of the follicular sheath in advanced cases. Conclusion Follicular microinflammation plays an integral role in the pathogenesis of Androgenetic Alopecia in early cases. Over time, thickening of perifollicular sheath takes place due to increased deposition of collagen, resulting in marked perifollicular fibrosis, and sometimes ends by complete destruction of the affected follicles in advanced cases.
http://www.biomediclaser.com/pdf/Inf...c-Alopecia.pdf
Formation of fibrous tissue or fibroplasia of the dermal sheath, which surrounds the hair follicle, is now suspected to be a common terminal process resulting in the
miniaturization. Involution of the pilosebaceous unit in this form of baldness and sustained microscopic
follicular inflammation with connective tissue remodeling, eventually resulting in permanent hair loss, is
considered a possible cofactor in the complex etiology of androgenetic alopecia. However, till date, the
inflammatory component has not been explored in developing treatment protocols for androgenetic
alopecia.
Fibrosing Alopecia in a Pattern DistributionPatterned Lichen Planopilaris or Androgenetic Alopecia With a Lichenoid Tissue Reaction Pattern?
Patients developed progressive fibrosing alopecia of the central scalp, without the multifocal areas of involvement typical of lichen planopilaris and pseudopelade. Perifollicular erythema, follicular keratosis, and loss of follicular orifices were limited to a patterned area of involvement. Biopsy specimens of early lesions demonstrated hair follicle miniaturization and a lichenoid inflammatory infiltrate targeting the upper follicle region. Advanced lesions showed perifollicular lamellar fibrosis and completely fibrosed follicular tracts indistinguishable from end-stage lichen planopilaris, pseudopelade, or follicular degeneration syndrome.
http://archderm.jamanetwork.com/article.aspx?articleid=189906
INFLAMMATORY PHENOMENA AND FIBROSIS
The implication of microscopic follicular inflammation in the pathogenesis of Androgenetic Alopecia has emerged from several independent studies: An early study referred to an inflammatory infiltrate of activated T cells and macrophages in the upper third of the hair follicles, associated with an enlargement of the follicular dermal sheath composed of collagen bundles (perifollicular fibrosis), in regions of actively progressing alopecia.[
25] Horizontal section studies of scalp biopsies indicated that the perifollicular fibrosis is generally mild, consisting of loose, concentric layers of collagen that must be distinguished from cicatricial alopecia.[
26] The term 'microinflammation' has been proposed, because the process involves a slow, subtle, and indolent course, in contrast to the inflammatory and destructive process in the classical inflammatory scarring alopecias.[
27] The significance of these findings has remained controversial. However, morphometric studies in patients with male pattern Androgenetic Alopecia treated with minoxidil showed that 55% of those with microinflammation had regrowth in response to treatment, in comparison to 77% in those patients without inflammation and fibrosis.[
26] Moreover, some forms of primary fibrosing alopecia may represent pathological exaggeration of Androgenetic Alopecia associated with follicular inflammation and fibrosis, specifically postmenopausal frontal fibrosing alopecia,[
28] and fibrosing alopecia in a pattern distribution.[
29]
An important question is how the inflammatory reaction pattern is generated around the individual hair follicle.
Inflammation is regarded as a multistep process which may start from a primary event. Some authors proposed that alopecia may result from cumulative physiological degeneration of selected hair follicles. They described in healthy murine skin clusters of perifollicular macrophages as perhaps indicating the existence of a physiological program of immunologically controlled hair follicle degeneration by which malfunctioning follicles are removed by programmed organ deletion, and suggested that perhaps an exaggerated form of this process might underlie some forms of primary scarring alopecia.[
30] The observation of a perifollicular infiltrate in the upper follicle near the infundibulum of human hair follicles in Androgenetic Alopecia suggests that the primary causal event for the triggering of inflammation might occur near the infundibulum.[
27] On the basis of this localization and the microbial colonization of the follicular infundibulum with
Propionibacterium sp.,
Staphylococcus sp.,
Malassezia sp., or other members of the transient flora, one could speculate that microbial toxins or antigens could be involved in the generation of the inflammatory response. Alternatively, keratinocytes themselves may respond to oxidative stress from irritants, pollutants, and UV irradiation, by producing nitric oxide, and by releasing intracellularly stored IL-1α. This pro-inflammatory cytokine by itself has been shown to inhibit the growth of isolated hair follicles in culture. [
31] Moreover, adjacent keratinocytes, which express receptors for IL-1, start to engage the transcription of IL-1 responsive genes: mRNA coding for IL-1β, TNFα, and IL-1α, and for specific chemokine genes, such as IL-8, and monocyte chemoattractant protein-1 (MCP-1) and MCP-3, themselves mediators for the recruitment of neutrophils and macrophages, have been shown to be upregulated in the epithelial compartment of the human hair follicle.[
27] Besides, adjacent fibroblasts are also fully equipped to respond to such a pro-inflammatory signal. The upregulation of adhesion molecules for blood-borne cells in the capillary endothelia, together with the chemokine gradient, drives the transendothelial migration of inflammatory cells, which include neutrophils through the action of IL-8, T cells, and Langerhans cells at least in part through the action of MCP-1. After processing of localized antigen, Langerhans cells, or alternatively keratinocytes, which may also have antigen presenting capabilities, could then present antigen to newly infiltrating T lymphocytes and induce T-cell proliferation. The antigens are selectively destroyed by infiltrating macrophages, or natural killer cells. On the occasion that the causal agents persist, sustained inflammation is the result, together with connective tissue remodeling, where collagenases, such as matrix metalloproteinase (also transcriptionally driven by pro-inflammatory cytokines) play an active role.[
27] Collagenases are suspected to contribute to the tissue changes in perifollicular fibrosis.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2929555/
Androgen Alopecia is a big cluster****.. and yeah the problem seems deeper that what most people think. Biopsies image vertical sectioning.
 - - - Updated - - -
- - - Updated - - -
Fibroplasia was noted, characterized by an increased number of fibroblasts and increased amounts of collagen around the sebaceous glands and collagenous streamers [ 9 ], in 62% of subjects (Figs. 4 , 5 and 6 ). .
http://www.springerimages.com/Images...3-003-0447-y-3
- - - Updated - - -
Scalp pathology in androgenetic alopecia: horizontal section. Severe peri-isthmus and peri-sebaceous lymphocytic infiltrates with perifollicular fibrosis (HE ×60)
http://www.springerimages.com/Images...3-003-0447-y-7
- - - Updated - - -
Scalp pathology in androgenetic alopecia: vertical section. Mild perifollicular fibrosis in the dermis (HE ×70)
http://www.springerimages.com/Images...3-003-0447-y-3
- - - Updated - - -
http://openi.nlm.nih.gov/detailedres...108-g015&req=4
Androgenetic Alopecia is characterized by progressive miniaturization of hair follicles. When the biopsy specimen is sectioned transversely at the level of opening of sebaceous ducts into the hair follicle, the hairs shafts appear vastly different in diameter. The position of the original terminal follicle is indicated by a follicular streamer (stellae or fibrous tract) extending from the subcutaneous tissue up to the course of the follicle to the miniaturized hair.
Decreased terminal hairs and increased follicular streamers therefore characterize Androgenetic Alopecia. Sebaceous glands seem enlarged in relation to the miniaturized hair follicles. There is significant reduction in total follicular counts, measured by horizontal sectioning of scalp biopsy. The progressive reduction in the duration of anagen causes a relative increase in telogen hair [Figures 13‐15].
- - - Updated - - -
Some good links for more biopsies pictures..
http://www.dermpedia.org/dermpedia-textbook/androgenic-alopecia
http://www.dermaamin.com/site/histop...alopecia-.html
Really cool biopsies from healthy and Androgenic Alopecia scalps...click on the link for the pics..
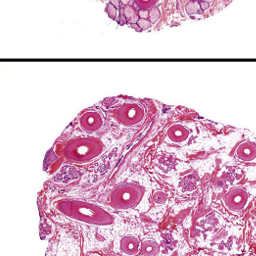 https://www.inkling.com/read/mckees-...on-of-alopecia
- - - Updated - - -
Histological features of peripilar signs associated with androgenetic alopecia.
Deloche C
https://www.inkling.com/read/mckees-...on-of-alopecia
- - - Updated - - -
Histological features of peripilar signs associated with androgenetic alopecia.
Deloche C,
de Lacharrière O,
Misciali C,
Piraccini BM,
Vincenzi C,
Bastien P,
Tardy I,
Bernard BA,
Tosti A.
Source
Centre Charles Zviak, L'Oréal Recherche, 90 rue du Général Roguet, 92583 Clichy Cedex, France.
Abstract
BACKGROUND:
A study of the scalp in a large cohort of volunteers with androgenetic alopecia using macrophotographs showed the presence of peripilar signs (PPS) around the hair ostia.
OBJECTIVE:
The aim of the present study was to establish the histopathological features related to PPS.
DESIGN:
Prospective clinicopathological study. SETTING. Department of Dermatology, University Hospital of Bologna.
PATIENTS:
A group of 40 patients (21 males and 19 females) participated in the study. Macrophotographs of the scalp were taken using a Dermaphot camera and PPS were scored using a three-point scale. Hair density and PPS were clinically scored according to reference scales. Two punch biopsies from the photographed area were obtained from each subject and histological analysis was performed on vertical and horizontal sections.
OBSERVATIONS:
Clinical parameters indicated that PPS were already detectable on scalp with high hair density. Moreover, in patients with high hair density (score >4), a significant relationship was found between the PPS score and the global score for perifollicular infiltrates. Thus PPS are linked to superficial perifollicular lymphocytic infiltrates in early androgenetic alopecia.
CONCLUSIONS:
PPS could be the clinical signs reflecting the presence of perifollicular infiltrates.
Scalp dermoscopy of androgenetic alopecia in Asian people.
Inui S,
Nakajima T,
Itami S.
Source
Department of Regenerative Dermatology, Graduate School of Medicine, Osaka University, Suita, Osaka, Japan.
Abstract
Although dermoscopy is used mainly for diagnosing pigmented skin lesions, this device has been reported to be useful in observing alopecia areata and frontal fibrosing alopecia. Herein, we investigated the dermoscopic features and their incidence of androgenetic alopecia (Androgenetic Alopecia; n = 50 men) and female Androgenetic Alopecia (***A; n = 10 women) in Asian people. More than 20% hair diameter diversity (HDD), which reportedly is an early sign of Androgenetic Alopecia and corresponds to hair follicle miniaturization, was observed in the affected area of all Androgenetic Alopecia and ***A cases, suggesting that HDD is an essential feature to diagnose Androgenetic Alopecia and ***A.
Peripilar signs, corresponding to perifollicular pigmentation, were seen in 66% (33/50) of Androgenetic Alopecia and 20% (2/10) of ***A women. This incidence in the present study was lower than previously reported in white subjects possibly because the Asian skin color conceals slight peripilar pigmentation. Yellow dots were observed in 26% (13/50) of Androgenetic Alopecia and 10% (1/10) of ***A cases and the number of yellow dots in Androgenetic Alopecia and ***A was limited to 10 on the overall hair loss area. Yellow dots possibly indicate the coincidence of Androgenetic Alopecia and enlargement of the sebaceous glands caused by common end-organ hypersensitivity to androgen. In conclusion, dermoscopy is useful to diagnose Androgenetic Alopecia and ***A and provides insights into the pathogenesis of Androgenetic Alopecia.
“During the hair cycle the follicle has to be rebuilt from stem cells,” explains Dr Bruno Bernard, director of research for life sciences at L’Oreal. “Stem cells in human hair follicles are localised in two different reservoirs – one is in the upper part of the follicle and the other in the lower part.
“The cells in the lower part are required to activate the cells in the upper part and so help to maintain the follicle function. The thickening of collagen in the connective tissue sheath, which sits around the base of the hair follicle, prevents the movement of stem cells from the lower reservoir to the upper reservoir. Bit by bit, the follicle is squeezed and causes the follicles to grow smaller and smaller.” Indeed, research from The Rockefeller University in New York suggests movement between the two groups of stem cells is crucial in normal hair growth.
http://www.telegraph.co.uk/science/8...he-corner.html
It is really important to go deep with the roller. [SIZE=-1][SIZE=-1][SIZE=-1][SIZE=-1][SIZE=-1]Perifollicular sheath is the major problem in Androgen Alopecia.[/SIZE][/SIZE][/SIZE][/SIZE][/SIZE]
 [SIZE=-1][SIZE=-1][SIZE=-1][SIZE=-1][SIZE=-1] http://dopingjournal.org/content/5/3/[/SIZE][/SIZE][/SIZE][/SIZE][/SIZE]
[SIZE=-1][SIZE=-1][SIZE=-1][SIZE=-1][SIZE=-1] http://dopingjournal.org/content/5/3/[/SIZE][/SIZE][/SIZE][/SIZE][/SIZE]